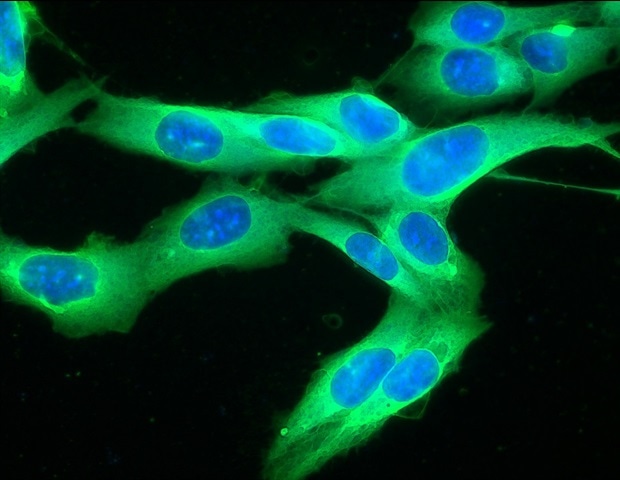
Like the surface of the moon, new research published today in Cell finds the existence of craters on the surface of melanoma cells that serve as immune hubs, becoming major sites for tumor killing. These craters could serve as good…
Blog
-
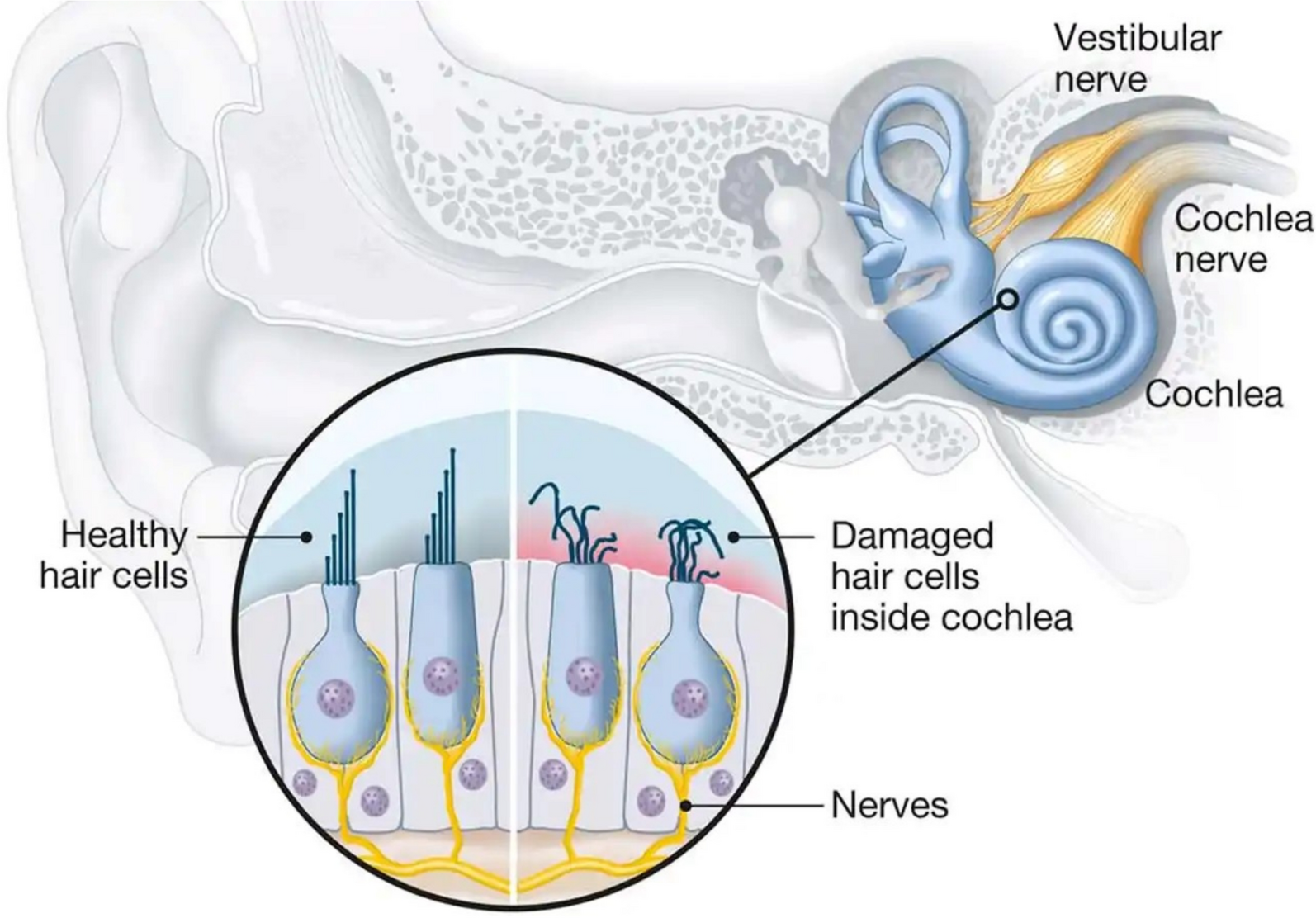
Audiological and radiological diagnosis of sensorineural hearing loss in Africa | Egyptian Journal of Radiology and Nuclear Medicine
Olusanya BO, Newton VE, Somefun AO (2014) Hearing impairment in children in developing countries. Lancet Glob Health 2:e4–e5. https://doi.org/10.1016/S2214-109X(13)70160-4
Google Scholar
Chin…
Continue Reading
-

The late Aga Khan’s last personal treasures go on sale
The late Aga Khan’s last personal treasures go on sale .1Findings showed that at a median follow-up of 16.56 months, the blinded independent review committee (BIRC)–assessed median PFS was 22.21 months (95% CI, 16.59-not reached [NR]) with fruquintinib plus sintilimab (n = 119) vs 6.90 months (95% CI, 5.55-8.31) with axitinib or everolimus (n = 115; HR, 0.373; 95% CI, 0.256-0.544; stratified log-rank P < .0001). The 12-month PFS rate was 69.0% in the combination arm vs 34.6% in the control arm, and the 18-month PFS rates were 54.6% vs 16.9%, respectively. Investigator-assessed median PFS was consistent with these findings at 16.59 months (95% CI, 13.80-NR) with fruquintinib plus sintilimab vs 5.82 months (95% CI, 5.49-8.28) with axitinib or everolimus (HR, 0.370; 95% CI, 0.260-0.527; stratified log-rank P < .0001). The 12- and 18-month investigator-assessed PFS rates for the experimental arm were 61.6% vs 27.4%, respectively; these respective rates were 47.9% vs 10.1% in the control arm.
“These results suggest that [fruquintinib plus sintilimab] should be a new choice for patients as second-line therapy for locally advanced and metastatic RCC,” Zhenhua Liu, MD, of West China Hospital at Sichuan University, in Chengdu, explained in the conclusion of his presentation.
What Was the Rationale and Design of the FRUSCIA-2 Trial?
Although TKI and immunotherapy combinations have become a standard of care in the first-line setting for patients with advanced RCC globally, Liu explained that TKI monotherapy has remained the first-line standard in China, leaving a key unmet need for patients who experience disease progression.
The phase 3 FRUSICA-2 trial was a randomized, open-label, active-controlled study that enrolled patients between 18 and 75 years of age with histologically or cytologically confirmed locally advanced or metastatic RCC who progressed on or were intolerant to first-line VEGFR TKI therapy. Additional inclusion criteria required an ECOG performance status of 0 or 1. Patients were excluded if they had received prior immune-modulatory therapy, except in the adjuvant or neoadjuvant setting without progression within 6 months of discontinuation.
A total of 234 patients were randomly assigned in a 1:1 ratio to receive fruquintinib plus sintilimab or axitinib or everolimus monotherapy. Patients in the experimental arm received fruquintinib at 5 mg orally once daily for 2 weeks on and 1 week off per 21-day cycle in combination with sintilimab at 200 mg intravenously once every 3 weeks. Those in the control arm received axitinib at 5 mg orally twice daily or everolimus at 10 mg orally once daily, both administered continuously in 21-day cycles.
Treatment continued until disease progression, death, intolerable toxicity, or another protocol-specified reason for discontinuation. Stratification factors included IMDC risk classification (favorable vs intermediate vs poor) and ECOG performance status (0 vs 1).
The primary end point was PFS by BIRC per RECIST 1.1 criteria. Secondary end points included investigator-assessed PFS, overall response rate (ORR), disease control rate (DCR), duration of response (DOR), time to response (TTR), and overall survival (OS).
What Were the Baseline Characteristics of the Patients in the Study?
The median age across both arms was 59.0 years (range, 36-75 in the fruquintinib plus sintilimab arm; 37-74 in the control arm). Patients 65 years of age or older accounted for 35.3% of those receiving fruquintinib plus sintilimab and 28.7% of those in the axitinib/everolimus group. Most patients were male, comprising 80.7% and 77.4% of each arm, respectively.
With respect to ECOG performance status, 42.0% of patients in the combination arm and 40.9% in the control arm had an ECOG performance status of 0, while 58.0% and 59.1%, respectively, had a performance status of 1. Similarly, the Karnofsky Performance Status of 100 or 90 was observed in 68.9% of patients in the combination arm and 72.2% in the control arm.
Based on IMDC risk classification, 27.7% vs 27.8% of patients were categorized as having favorable risk, 61.3% vs 62.6% as intermediate risk, and 10.9% vs 9.6% as poor risk in the fruquintinib plus sintilimab and control groups, respectively. Approximately two-thirds of patients in each group had 0 to 1 IMDC risk factors (63.9% vs 63.5%), and the remainder had two or more (36.1% vs 36.5%).
The majority of patients (73.9% in the fruquintinib plus sintilimab arm and 58.3% in the control arm) had 2 or more organs with metastatic involvement. PD-L1 expression, defined as a combined positive score (CPS) of at least 1, was reported in 19.3% of patients receiving fruquintinib plus sintilimab and 17.4% in those treated with axitinib or everolimus, and PD-L1 CPS less than 1 was seen in 47.1% and 46.1%, respectively. The remainder had unknown PD-L1 status.
Sarcomatoid or rhabdoid differentiation features were identified in a small subset of patients—4.2% in the fruquintinib plus sintilimab arm and 8.7% in the control arm. All patients had received prior VEGFR-targeted therapy, most commonly sunitinib (Sutent; 52.1% vs 56.5%) or pazopanib (36.1% vs 34.8%), with smaller proportions having received sorafenib (Nexavar) or axitinib. Prior nephrectomy was performed in 81.5% of patients in the fruquintinib plus sintilimab arm and 80.0% in the control arm.
What Additional Efficacy and Response Findings Were Observed in the Trial?
Per BIRC, the ORR was 60.5% (95% CI, 51.13%-69.34%) with fruquintinib plus sintilimab vs 24.3% (95% CI, 16.83%-33.23%) with axitinib or everolimus (odds ratio [OR], 4.622; P < .0001). The DCR was 90.8% vs 88.7%, respectively, and the median DOR was 23.69 months (95% CI, 14.46-NR) vs 11.33 months (95% CI, 6.90-NR). Median TTR was 2.79 months with fruquintinib plus sintilimab and 2.69 months with axitinib or everolimus.
Investigator-assessed outcomes mirrored the BIRC results, with an ORR of 62.2% (95% CI, 52.84%-70.91%) for the combination vs 27.8% (95% CI, 19.87%-36.95%) for standard therapy (OR, 4.192; P < .0001). The DCR was 90.8% vs 87.8%, and the median DOR was 17.97 months (95% CI, 13.83-NR) vs 11.04 months (95% CI, 4.17-12.45).
At the time of data cutoff, OS data reached approximately 20% maturity.
What Safety Findings Were Observed in the Trial?
According to Liu, the safety profile of fruquintinib plus sintilimab in the FRUSICA-2 trial was manageable and consistent with the known toxicities of VEGFR inhibitors and immune checkpoint blockade. Treatment-emergent adverse effects (TEAEs) occurred in all patients across both study arms.
Grade 3 or higher TEAEs were reported in 71.4% of patients receiving fruquintinib plus sintilimab compared with 58.8% of those treated with axitinib or everolimus. Treatment-related AEs (TRAEs) occurred in 100% of patients in the fruquintinib plus sintilimab arm and in 99.1% of those in the control arm, with grade 3 or higher TRAEs observed in 59.7% and 48.2% of patients, respectively. Serious TEAEs were more frequent in the combination group (48.7%) than in the control group (29.8%).
Immune-related AEs (irAEs), assessed by investigators, were reported in 63.0% of patients in the fruquintinib plus sintilimab arm, with 27.7% experiencing grade 3 or higher effects. Infusion-related reactions (IRRs) occurred in 1.7% of patients treated with the combination regimen.
The most common TEAEs reported in at least 20% of patients in either group included proteinuria, hypothyroidism, palmar-plantar erythrodysesthesia syndrome, hypertension, hypertriglyceridemia, elevated blood creatinine levels, decreased weight, asthenia, anemia, diarrhea, and elevations in liver function enzyme levels. Rates of hypothyroidism, hypertension, and proteinuria were slightly higher in the combination arm, whereas diarrhea and weight loss occurred more frequently in patients treated with axitinib or everolimus.
China’s National Medical Products Administration is currently evaluating a new drug application seeking approval of fruquintinib with sintilimab for patients with previously treated locally advanced or metastatic RCC, supported by data from the FRUSICA-2 trial.2
References
- Ye D, He Z, Qu Z, et al. Fruquintinib (FRUQ) plus sintilimab (SIN) versus axitinib (AXI) or everolimus (EVE) monotherapy as 2L treatment in pts with locally advanced or metastatic renal cell carcinoma (RCC): Results from phase III part of a randomized, open-label, active-controlled phase II/III study (FRUSICA-2). Presented at: 2025 ESMO Congress; October 17-20, 2025; Berlin, Germany. Abstract 2592MO.
- HUTCHMED highlights FRUSICA-2 registration trial data to be presented at the 2025 ESMO Congress. News release. HUTCHMED. October 13, 2025. Accessed October 17, 2025. https://www.hutch-med.com/esmo25-frusica2/
Continue Reading
-

2025 Daytime Emmys: Complete Winners List
General Hospital dominated the 2025 Daytime Emmy Awards with seven total wins, while Sir David Attenborough broke a record that was previously set just one year earlier.
Attenborough notably beat Dick Van Dyke’s record as the
Continue Reading
-

Beautiful work by Conor W O’Brien offers truly awesome facts – The Irish Times
Late Learner by Ciara Geraghty (HarperCollins, £14.99)
“Learning to drive did as much good for my mental health as four years of therapy” – it’s this admission, made by a close friend, that springs to mind when I read the blurb of…
Continue Reading
-
Review reveals how paternal lifestyle shapes sperm epigenetics and offspring health
A new review in Clinical Epigenetics synthesises growing evidence that paternal lifestyle and environmental exposures such as diet, obesity, smoking, endocrine-disrupting chemicals, and stress alter sperm epigenetic marks (DNA…
Continue Reading
-
The TLP issue – Dawn
- The TLP issue Dawn
- Punjab says it’ll recommend a ban on TLP. Here’s what that means Dawn
- Violence erupts in eastern Pakistan as Islamists try to march on capital for pro-Palestinian rally AP News
- TLP offices sealed in Islamabad after violent…
Continue Reading